Bern, 24.11.2023 – Human research in Switzerland: A review of 2022. Following the challenges of the COVID-19 pandemic, the number of research projects in Switzerland returned to normal and was close to pre-pandemic levels.
Reports on the activities of the ethics committees and human research
The report on the activities of the ethics committees and human research in 2022 provides a detailed insight into developments and activities in the area of human research in Switzerland.
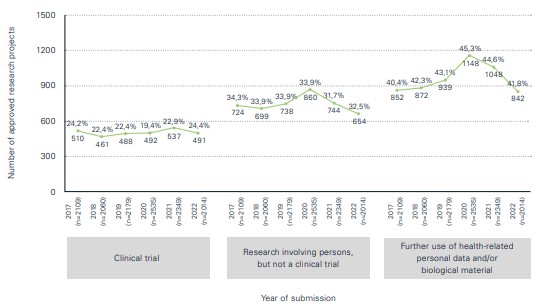
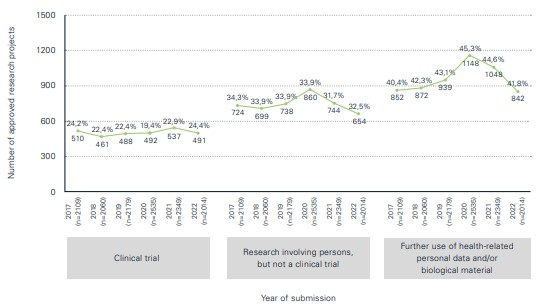
In summary, following the many different challenges of the COVID-19 pandemic, the number of research projects reviewed and approved by the seven ethics committees in Switzerland returned to normal and was only slightly below pre-pandemic levels.
Despite the post-pandemic normalisation, the ethics committees are seeing tough challenges emerging in various areas. These include handling of research with medical devices in a European context as well as complex issues relating to digitalisation in research, with regard to artificial intelligence, big data and decentralised clinical trials in particular.
Overall, the report makes it clear that Switzerland continues to play an active role in human research. The ethics committees have a central role in ensuring ethical and legal standards.
The "Statistical Report – Human Research in Switzerland 2022" confirms the return to normal of the number of studies to pre-COVID-19 levels. The results of the statistical analyses, compiled in collaboration with swissethics and the Department of Clinical Research at the University of Basel on the basis of data from the Business and Administration System for Ethics Committees (BASEC), show that:
- Last year, a total of 2,407 clinical trials were submitted and 2,014 trials were approved. In view of the continuing decline in COVID-19-related research applications to the ethics committees, the decision was taken to no longer distinguish COVID-19-specific applications in the tables and figures.
- The most applications were submitted to the Zurich ethics committee, followed by the Ethics Committee of Northwestern and Central Switzerland and the Commission cantonale d'éthique de la recherche sur l'être humain (CER-VD).
Coordination Office for Human Research
Since the Human Research Act entered into force in 2014, the Coordination Office for Human Research (kofam) at the FOPH has been reporting annually on the activities of the cantonal research ethics committees and other supervisory authorities that are involved in the approval process for human research projects.
In addition, since 2016 an annual statistical overview has been compiled in conjunction with swissethics (the umbrella organisation of the cantonal ethics committees) showing the type and number of research projects. kofam coordinates the supervisory authorities and informs the public about their activities. It has its own website at www.kofam.ch.
Last modification 24.11.2023
Contact
Federal Office of Public Health FOPH
Communication and Campaigns Division
Schwarzenburgstrasse 157
3003
Bern
Switzerland
Tel.
+41 58 462 95 05