Bern, 21.12.21 – The coronavirus pandemic has also left its mark on human research in Switzerland, as illustrated by the activity report of the research supervisory authorities and the statistical report on human research for 2020.
Reports on the activities of the ethics committees and human research
Some (pandemic-related) features compared with previous years
The coronavirus pandemic had an impact on human research projects in 2020, as shown by the activity report of the ethics committees. Besides the increased volume of research projects submitted for review, the cantonal ethics committees also encountered the following specific features:
- switch to remote working from home;
- the associated challenges and solutions in examination and approval practice (e.g. online circulation processes for efficient application handling);
- approval of an eConsent approach for facilitated granting of electronic (instead of paper-based) consent by subjects in difficult epidemiological circumstances;
- processing times for examining and approving applications, which were maintained compared with previous (pre-pandemic) years, and in the case of coronavirus-related applications, were even significantly reduced.
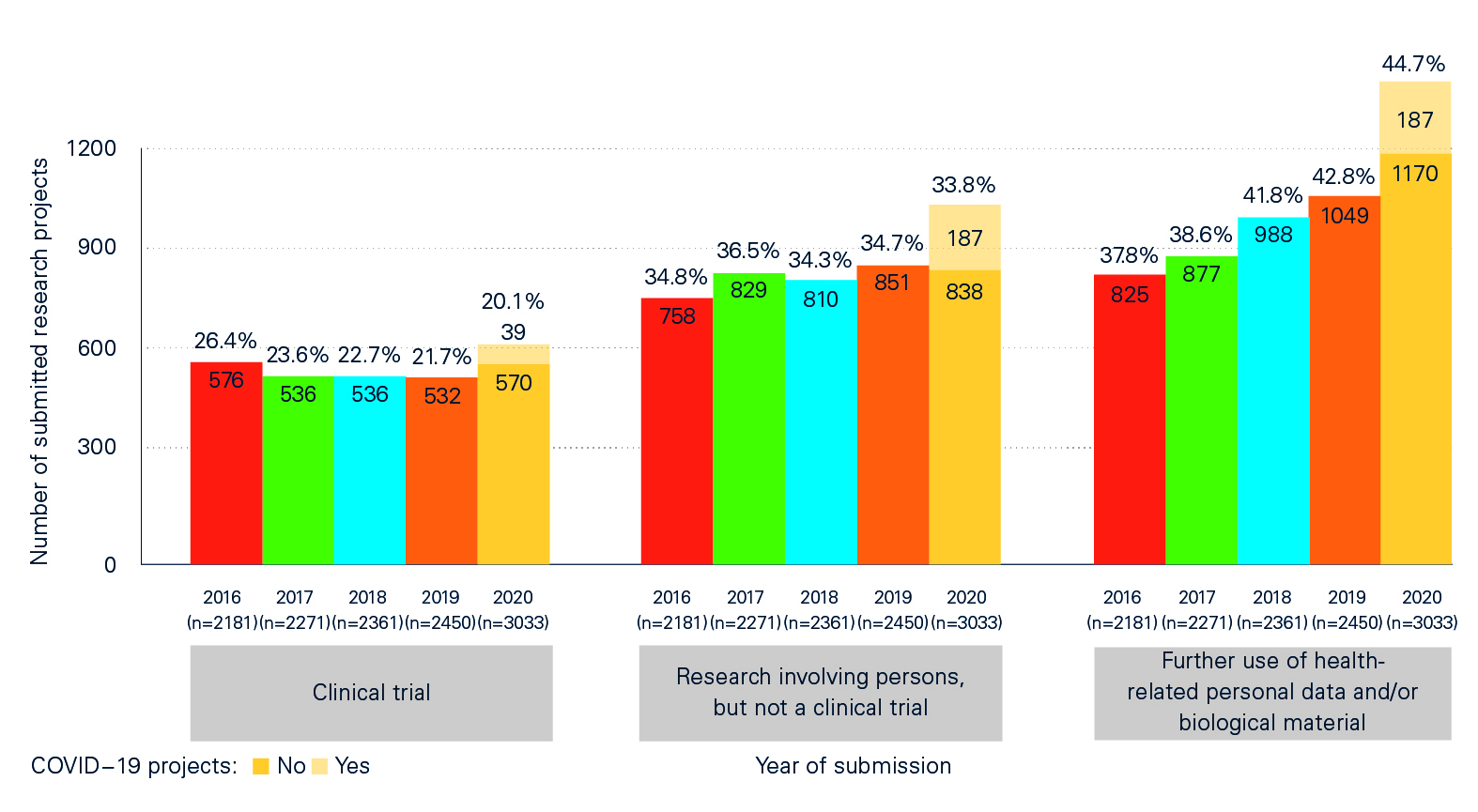
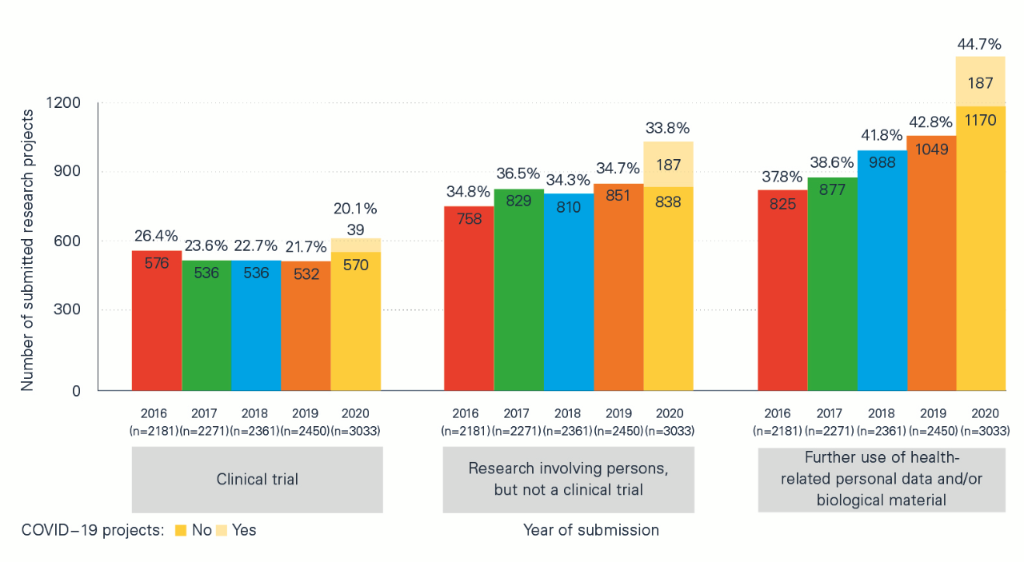
The impact of the pandemic can also be seen in the ‘Statistical report on Human Research in Switzerland 2020’, which contains detailed information and comparisons between COVID-19 projects and non-COVID-19 projects and between the pandemic year of 2020 and previous years. The analyses compiled in collaboration with swissethics and the Basel Clinical Trials Unit (CTU) on the basis of BASEC data (Business and Administration System for Ethics Committees) show, among other things, that:
- of a total of 3,033 applications submitted in 2020, 420 (just under 14%) were for COVID-19-related or SARS-CoV-2-related projects;
- the ethics committees in French- and Italian-speaking Switzerland received proportionally more COVID-19 applications than the other ethics committees, and that the ethics committee in Ticino saw the total number of applications submitted double compared with the previous year;
- in the month following the first confirmed SARS-CoV-2 infection in Switzerland, a significant number of projects were submitted, and this cluster of applications did not decline again until August 2020;
- the larger number of applications compared with previous years – there were 24% more applications than in 2019 – was not only due to research projects related to COVID-19 or SARS-CoV-2, but to other, non-COVID-19-related projects.
Coordination Office for Human Research
Since the Human Research Act entered into force in 2014, the Coordination Office for Human Research (kofam) at the FOPH has been reporting annually on the activities of the cantonal research ethics committees and other supervisory authorities that are involved in the approval process for human research projects. In addition, since 2016 a statistical overview has been compiled in conjunction with swissethics (the umbrella organisation of the cantonal ethics committees) showing the type and number of research projects.
Last modification 21.12.2021
Contact
Federal Office of Public Health FOPH
Division of Biomedicine
Human Research Section
Schwarzenburgstrasse 157
3003
Bern
Switzerland
Tel.
+41 58 463 51 54